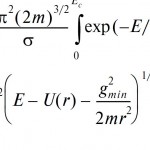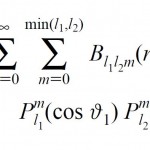
Theoretical Chemistry Group of N.I. Lobachevsky State University of Nizhny Novgorod, 23 Gagarin Avenue, 603950 Nizhny Novgorod, Russia
E-mail: tcg@ichem.unn.ru
The group is a part of the Scientific-Educational Center (SEC) “Modern methods of photochemistry, quantum chemictsry and spectroscopy for the research and development of substances, structures, and processes”
The group is focused on quantum chemical studies of reaction mechanisms and atomistic simulations of physicochemical processes with an emphasis on catalytic and photocatalytic reactions, surface phenomena, and the processes involving biomolecules.
Have a look at some interesting results obtained by us (click on miniature to enlarge the figure):
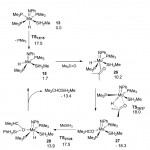
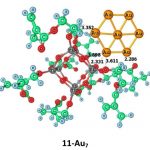 The structural, thermodynamic and spectral properties of photocatalytically active composite copolymers of polytitaniumoxide and hydroxyethyl methacrylate (HEMA) with incorporated gold nanoparticles TiO2/HEMA/Au have been studied. These new materials exhibits pronounced photocatalytic properties and we show that these properties can be tuned or controlled using the material structure and even the irradiation wavelength.
The structural, thermodynamic and spectral properties of photocatalytically active composite copolymers of polytitaniumoxide and hydroxyethyl methacrylate (HEMA) with incorporated gold nanoparticles TiO2/HEMA/Au have been studied. These new materials exhibits pronounced photocatalytic properties and we show that these properties can be tuned or controlled using the material structure and even the irradiation wavelength.
For more details see: [Comp.Theor.Chem., 2017, 1118, 1–15]
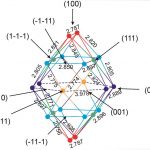 Adsortpion and diffusion of atomic and molecular hysdrogen on the surface of Pt24 subnanoparticle were studied. This system is a model for very efficient nanocatalysts in hydrogenation reactions. Adsorption of H2, its disscoiation to H atoms, surface migration of H on the surface, subsurface diffusion were considered. The full energetic map for the surface coordination of H was obtained. All the local minima and transition states between them were located and characterized.
Adsortpion and diffusion of atomic and molecular hysdrogen on the surface of Pt24 subnanoparticle were studied. This system is a model for very efficient nanocatalysts in hydrogenation reactions. Adsorption of H2, its disscoiation to H atoms, surface migration of H on the surface, subsurface diffusion were considered. The full energetic map for the surface coordination of H was obtained. All the local minima and transition states between them were located and characterized.
For more details see: [J.Phys.Chem.C, 2016, 120(33), 18570–18587]
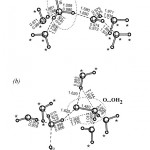
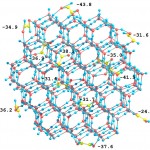 Adsortpion of glyoxal (HOC-COH) molecules at the atmospheric nanoparticle of water ice (270 water molecules and 12 glyoxal molecules). The ice particle of such a size undergoes spontaneous crystallization. Full optimization by DFTB method. Values are the adsorption energies at the corresponding sites.
Adsortpion of glyoxal (HOC-COH) molecules at the atmospheric nanoparticle of water ice (270 water molecules and 12 glyoxal molecules). The ice particle of such a size undergoes spontaneous crystallization. Full optimization by DFTB method. Values are the adsorption energies at the corresponding sites.
For more details see: [J.Phys.Chem.C, 2014, 118(14), 7398–7413]
The mechanism of carbonyl hydrosilylation by silyl hydride complex [(ArM=)Mo(H)(SiH2Ph)(PMe3)3]. The theoretical exploration of the reaction mechanism along with the experimental studies (carried out in the group of Prof. G.I. Nikonov from Brock Univeristy, Canada) revealed the catalytic cycle allowing to use such a complex for the fine synthesis of organosilicon compounds.
For mode details see: [Chem.Eur.J., 2013, 19(26), 8573–8590]
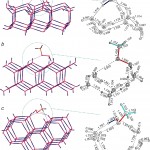 Adsorption of methylhydroperoxide (MHP, CH3OOH) at the crystalline surface of hexagonal water ice Ih. MHP is one of the most active atmospheric oxidants participating in the oxidation of many atmospheric molecules. Optimization of clusters (H2O)72-MHP at the BLYP/6–31+G(d,p) level.
Adsorption of methylhydroperoxide (MHP, CH3OOH) at the crystalline surface of hexagonal water ice Ih. MHP is one of the most active atmospheric oxidants participating in the oxidation of many atmospheric molecules. Optimization of clusters (H2O)72-MHP at the BLYP/6–31+G(d,p) level.
For more details see: [J.Phys.Chem.C, 2011, 115(18), 9081–9089]
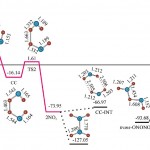 PES profile of the nitric oxide oxidation 2 NO + O2 = 2 NO2. Full optimization at the CCSD(T)/cc-pVDZ and CAS(26,16)/cc-pVDZ levels.
PES profile of the nitric oxide oxidation 2 NO + O2 = 2 NO2. Full optimization at the CCSD(T)/cc-pVDZ and CAS(26,16)/cc-pVDZ levels.
For more details see: [J.Chem.Theor.Comp., 2011, 7(7), 2021–2024]
Exact expression for the classical partition function of the bound and quasi-bound states of the weakly bound gas-pase dimers formed by the nonpoint monomers. This make it possible to calculate accurately the thermodynamic functions and equilibrium constants for the weakly-bonded complexes in the gas phase including many practically important atmospheric trace gases.
For more details see: [Rus.J.Phys.Chem.B, 2010, 4(1), 44–52]
Structure and properties of oxywater H2OO formed at the water ice surface and inside the ice during the UV ozone photolysis.
For more details see: [Phys.Chem.Chem.Phys., 2003, 5, 496–505]
Bipolar expansion of the Ohno potential. This is the fundamental mathematical relationship which was earlier known for the Coulomb potential (obtained by Buehler and Hisrschfelder in 1951). Now, the B coefficients are written out for the damped Coulomb interaction (Ohno potential) allowing the efficient calculations of the two-center molecular integrals arising in the modern NDDO-based semiempirical methods .
For more details see: [J.Phys.Chem., 1996, 100(15), 6354–6358]
Have a look at our software for quantum chemical studies including Moltran